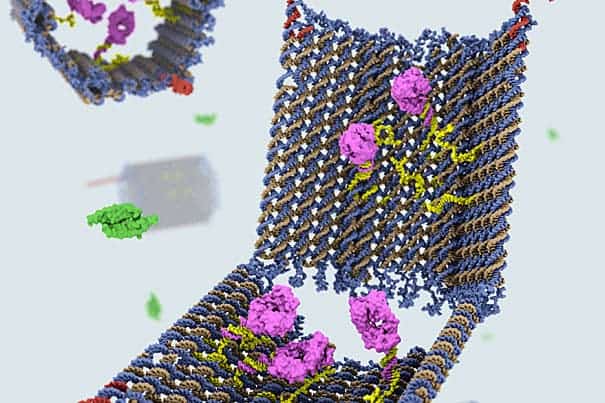
In what can only be hailed as a breakthrough in the “smart drugs” field, scientists at Harvard University have successfully managed to create nanorobots made out of strands of DNA, folded together by the DNA origami method. These act like drug-carrying recipients, which specifically target various types of cells and deliver complex molecular instructions – like telling cancer cells to self-destruct.
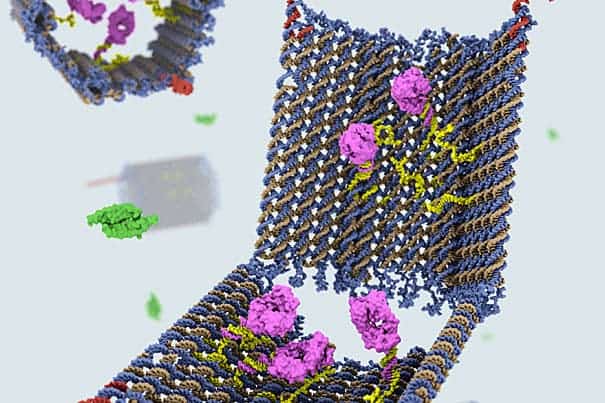
The shape and structure of the nanobots was critical to their success. The team designed a clam-like device, using DNA modelling software that can compute and complement inputted shapes with the right kinds of DNA strands, of the right helical structure and base pairs, and mix them together.
The DNA clam acts as a container and only opens when it finds its target. To keep its payload unscathed, made out specific molecules with encoded instructions for certain cell surface receptors with which it interacts, the clam is fitted with two locks. Each lock is made out of a DNA strand called an aptamer, specifically designed to recognize a certain molecule. Only when it nears the target, will the aptamer unzip, swinging the claim open, and delivering the payload in the process.
The scientists involved in research were Shawn Douglas, Ph.D., a Wyss Technology Development (Harvard University) Fellow, and Ido Bachelet, Ph.D., a former Wyss Postdoctoral Fellow who is now an Assistant Professor in the Faculty of Life Sciences and the Nano-Center at Bar-Ilan University in Israel.
To demo their creation, Douglas and Bachelet encoded antibody fragments with self-termination instructions for two types of cancer cells – leukemia and lymphoma. Since the two cancer cells communicate differently, they require specific instructions of their own, so the researchers were sure to have the messages written in different antibody combinations.
Smart DNA robots – miracle drugs of the future?
The nanorobot for leukemia had its locks open in response to molecules expressed on the cancer cells surface, and was loaded with a single molecules which kills cells by disrupting their growth cycles. Millions of such bots were released into a mixture of both healthy and cancerous human blood cells. Only three days afterwards, half of all the cancer cells were destroyed, while absolutely no healthy cells were affected at all. The researchers claim had they increased the number of payloads into the system, then every leukemia cell would’ve been cleansed.
What’s important to note about this particular system, whose design was heavily influenced by our own natural immune system, is that the active molecules designed to attack a specific cell can be harbored into containers featuring two types of locks. Just like the body’s immune system, the DNA origami nanobots will thus be able to hone in on specific cells in distress, bind to them, and transmit comprehensible signals to them to self-destruct. “It would require that two different signals have to be present to open it, increasing its specificity,” says Douglas.
“This work represents a major breakthrough in the field of nanobiotechnology as it demonstrates the ability to leverage recent advances in the field of DNA origami pioneered by researchers around the world, including the Wyss Institute’s own William Shih, to meet a real-world challenge, namely killing cancer cells with high specificity,” said Wyss Institute Founding Director, Donald Ingber, M.D., Ph.D. “This focus on 9translating technologies from the laboratory into transformative products and therapies is what the Wyss Institute is all about.”
By all standards, this can only be considered a remarkable research, with potentially incredible consequences in medicine. Thoughts, please?