The Origin of the Nitroplast
In a never-before-seen finding, scientists have recorded a rare evolutionary advancement in which an algae species has assimilated a cyanobacterium, blending into a single organism. During this process, the cyanobacterium has transformed into an organelle. This occurrence, known as primary endosymbiosis, has turned the cyanobacterium into a functioning part of the algae, similar to how ancient microbes evolved into mitochondria and chloroplasts, the generators of energy in today’s plants and animals.
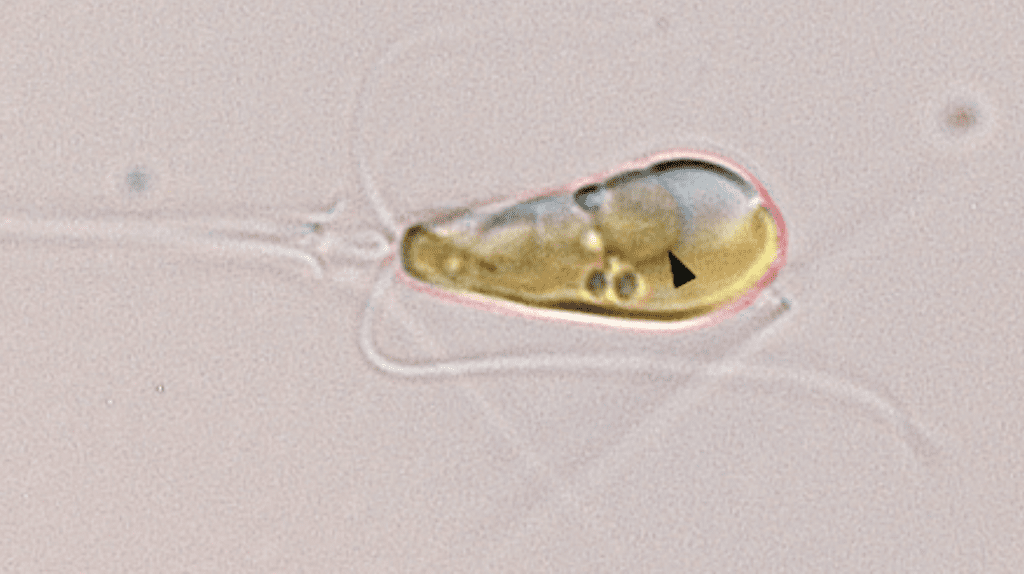
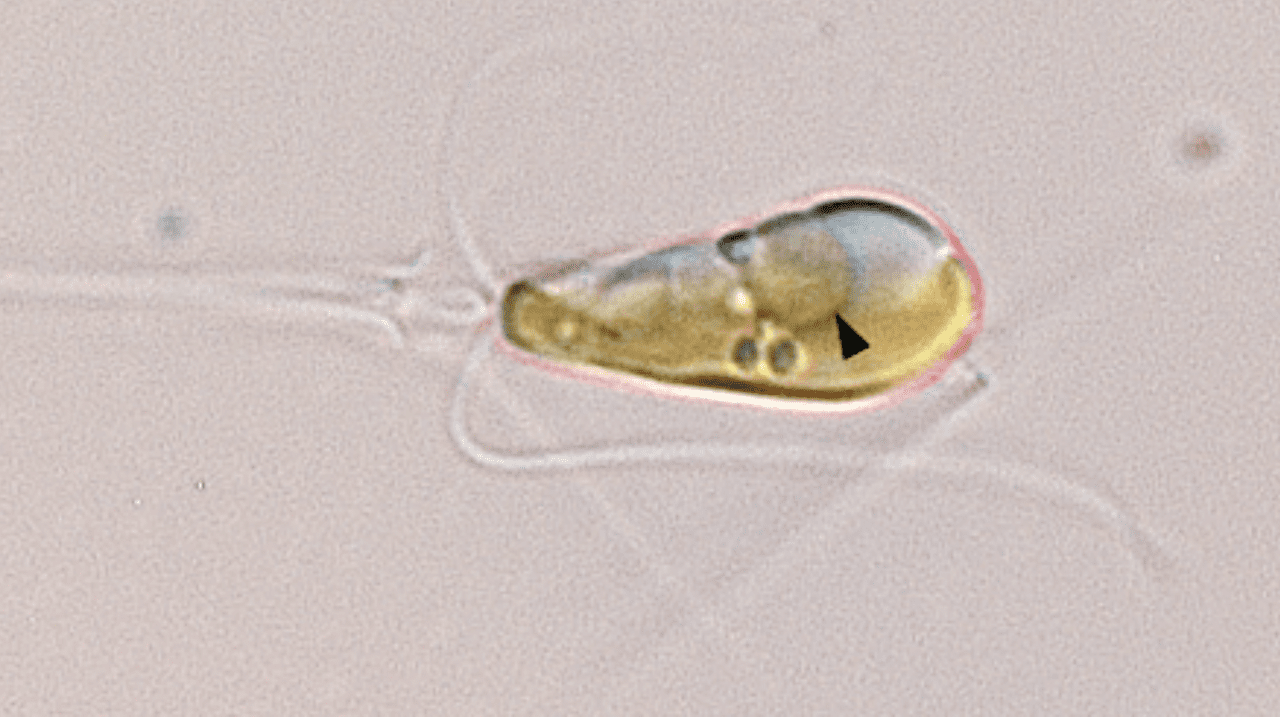
This new organelle, named a 'nitroplast', is only the fourth documented case of primary endosymbiosis, where a smaller prokaryotic cell is assimilated by and becomes a crucial part of a larger eukaryotic cell. The new single organism joined together from two entities possesses the impressive ability to extract nitrogen directly from the air, a capability that its predecessors could not accomplish. The last time a primary endosymbiosis event was witnessed by evolution, as far as we know, was when plants emerged.
Nitroplasts evolved from a cyanobacterium called Atelocyanobacterium thalassa (UCYN-A) becoming absorbed by the unicellular algae Braarudosphaera bigelowii. This event took place just 100 million years ago, as concluded by the researchers recently.
What this indicates is that, unlike typical symbiotic relationships where plants depend on external bacteria for nitrogen fixation, in B. bigelowii, this capability is internalized. Previously, only some prokaryotes similar bacteria were thought to have this ability. B. bigelowii is, as of now, the only eukaryote able to convert nitrogen to ammonia independently.
The Meandering Path of Discovery
The path to this finding was not fast or direct. In 1998, Jonathan Zehr, a marine sciences professor at UC Santa Cruz, stumbled upon a DNA sequence implying the existence of a then-unknown nitrogen-fixing cyanobacterium in the Pacific Ocean. This marked the start of a journey involving numerous researchers across continents.
Kyoko Hagino, a paleontologist from Kochi University in Japan, encountered a similar challenge as she painstakingly attempted to cultivate a species of marine algae. This species would eventually turn out to be the host for the mysterious organism UCYN-A. Her success, after over 300 previous failed attempts, allowed scientists to study UCYN-A and its interactions with its host in the lab.
According to the research, the connection between UCYN-A and its algal host is characterized by a synchronized exchange of nutrients. Their metabolisms are linked.
“That’s exactly what happens with organelles,” said Zehr. “If you look at the mitochondria and the chloroplast, it’s the same thing: they scale with the cell.”
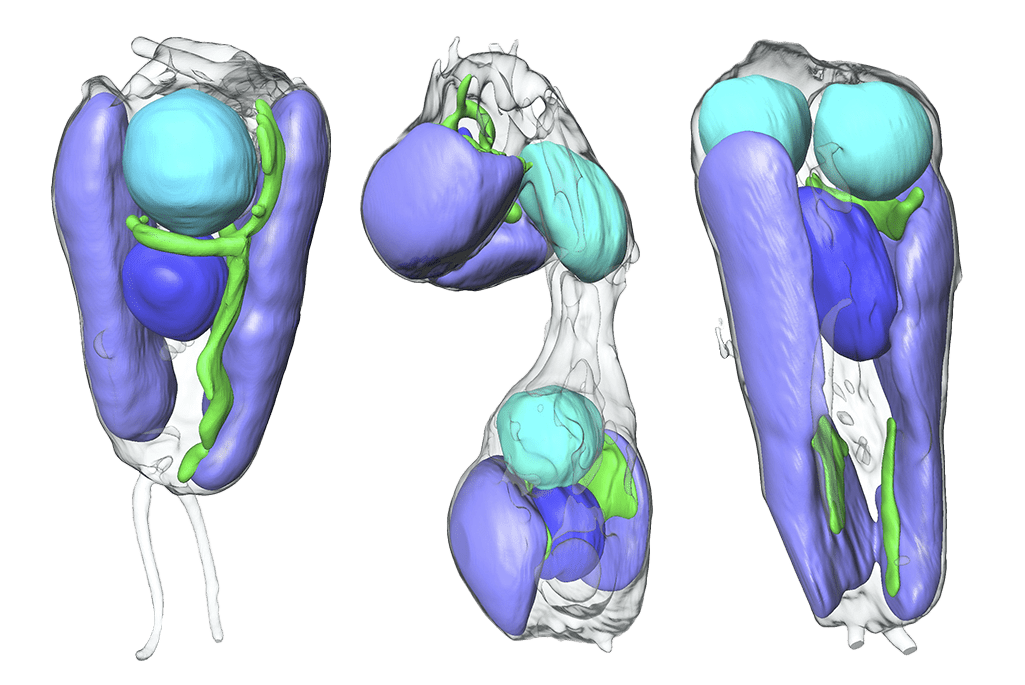
Additional convincing proof was provided by advanced imaging techniques developed by researchers at Berkeley Lab. Using a gentle X-ray imaging method, the scientists vividly captured the organelle’s movements during cell division. The images revealed that UCYN-A is inherently connected with the algal cell’s process.
In conclusion, the researchers studied the protein compositions of UCYN-A. They found that around half of the proteins in UCYN-A come from the algae host. These host proteins have a specific amino acid sequence that directs the cell to transport them into the nitroplast.
“That’s one of the hallmarks of something moving from an endosymbiont to an organelle,” said Zehr. “They start throwing away pieces of DNA, and their genomes get smaller and smaller, and they start depending on the mother cell for those gene products — or the protein itself — to be transported into the cell.”
The newest organelle in nature
The results of these long-term and international efforts, which were recently published in Cell and Science, demonstrate how UCYN-A has transformed from simply being closely associated with an alga, to becoming a fundamental part of its cellular structure — an organelle.
The appearance of the nitroplast is only the fourth known instance of primary endosymbiosis. The other three instances are mitochondria (thought to have originated from the engulfment of aerobic bacteria by an ancestral eukaryotic cell), chloroplasts (originating from a eukaryotic host cell engulfing a photosynthetic cyanobacterium), and another chloroplast-like structure called a chromatophore. These organelles developed over a billion years ago.
The nitroplast is not only essential for enhancing our understanding of cellular evolution but also has significant implications for broader ecological and agricultural systems.
The production of ammonia-based fertilizers from atmospheric nitrogen, which began in the early 20th century through the Haber-Bosch process, greatly increased agricultural productivity and global population growth. However, this process is also responsible for about 1.4% of worldwide carbon emissions. For years, scientists have been exploring ways to utilize natural nitrogen fixation in agriculture to reduce dependence on industrial processes. The nitroplast and its abilities could pave the way for new, sustainable agricultural technologies.
“This system is a new perspective on nitrogen fixation, and it might provide clues into how such an organelle could be engineered into crop plants,” said Tyler Coale, a postdoctoral scholar at UC Santa Cruz and first author of the new study.