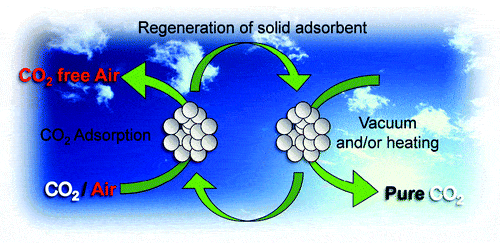
In a remarkable feat of engineering, scientists have come a huge step closer to achieving what’s maybe the greatest green energy dream ever. University of Southern California researchers have developed the world’s currently most effective CO2 absorbent material, which could render extraordinary results for the development of large scale batteries or whole CO2 absorbing parks, acting like “artificial trees”. After being collected the CO2 can be combined hydrogen to generate methanol fuel. If this wasn’t enough, the solution developed by the researchers is also the cheapest available at the moment.
The researchers, led by George Olah, a chemist at the University of Southern California (USC) in Los Angeles, was geared to developing a CO2 absorbent that is both effective and cheap to manufacture – they hit it all the way. As fossil fuels become ever scarce, one of the interesting proposed solutions is methanol fuel, which Olah, who was awarded the Nobel prize for chemistry in 1994, suggest could be derived from the collected CO2 combined with hydrogen stripped from water.
To achieve their goals, the scientists used polyethylenimine (PEI), a cheap polymer that is a decent COabsorber. Because of its limited absorbing surface, the PEI has moderate performances, so they decided to fuse it to a silica substrate, an industrially produced porous solid, which vastly increases its surface area, and thus its effectiveness.
As part of a demo, the researchers tested the material in normal humid conditions and observed each gram of the material sopped up an average of 1.72 nanomoles of CO2. To get an idea, that’s well above the 1.44 nanomoles per gram absorbed by a similar solution made by aminosilica, which had one of the best previous results and is a lot more costly.
While artificial trees or methanol fuel generators are incredibly appealing prospects, it will probably take a long time before this kind of technology becomes integrated to the necessary scale. However, an immediate application might arise much sooner. Olah plans to incorporate the material in iron-based battery for renewable energy storage for energy grids. Iron-based batteries are used to store the excess energy produced by wind turbines or solar cells, and to function the batteries grab oxygen from the air. That’s all fine and dandy, however when they interact with CO2, the battery risks severe malfunction. So far, the industry has used porous solids called zeolites and metal organic frameworks to coat the batteries and protect them from CO2, however these are extremely expensive. Yes, the ground has been set for the polyethylenimine based sponge.
Once saturated with CO2, the PEI-silica combo is easy to regenerate. The CO2 floats away after the polymer is heated to 85°C and can also catalyze at room temperature. “This is intriguing. It’s nice that it works at low temperatures,” says Klaus Lackner, a CO2 air-capture expert at Columbia University.
The team’s findings and research was presented in last month’s Journal of the American Chemical Society.
source via inhabitat