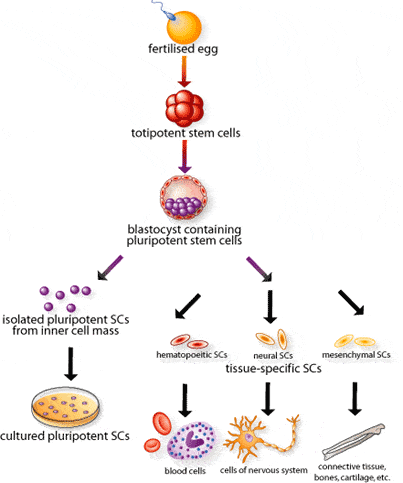
In was is a historic day, US doctors have officially begun the first trial of using human embryonic stem cells, as a result of the green light they got from regulators. The controversial method has been on the table for quite a while, and now The Food and Drug Administration has given a license to Geron to use stem cells to treat people with spinal injuries. These cells have the potential of becoming virtually any cell needed, including nerve cells.
Geron, the corporation who will lead this pivotal research is based in “silicon valley”, has spent 170 million dollars to develop a treatment for spinal cord injuries and reported that after the stem cell treatment, paralyzed rats regained some of their movement. However, it is still not yet clear how well people will respond to this kind of treatment; researchers are optimistic and they have already started human trials.
“When we started working with human embryonic stem cells in 1999, many predicted that it would be a number of decades before a cell therapy would be approved for human clinical trials. This accomplishment results from extensive research and development and a succession of inventive steps.”
And a great accomplishment it is ! Hopefully, this will be just the first step, and stem cells will be applied in other sorts of clinical trials too, because the huge potential they have cannot be neglected. Still, it will take some time to see results.
Professor Sir Ian Wilmut, director of the Medical Research Council Centre for Regenerative Medicine at the University of Edinburgh, said:
“This is very exciting news, however, it is very important to appreciate that the objective of trials at this stage is to confirm first of all that no harm is done to patients, rather than to look for benefits. Once that has been confirmed then the focus moves on to development and assessment of the new treatment.”
“This is indeed a significant milestone in our journey towards the promise of stem cell-based medicines. The global stem cell and regenerative medicine community will be awaiting the results of this safety trial with much anticipation.”, added Ben Sykes, executive director of the UK National Stem Cell Network